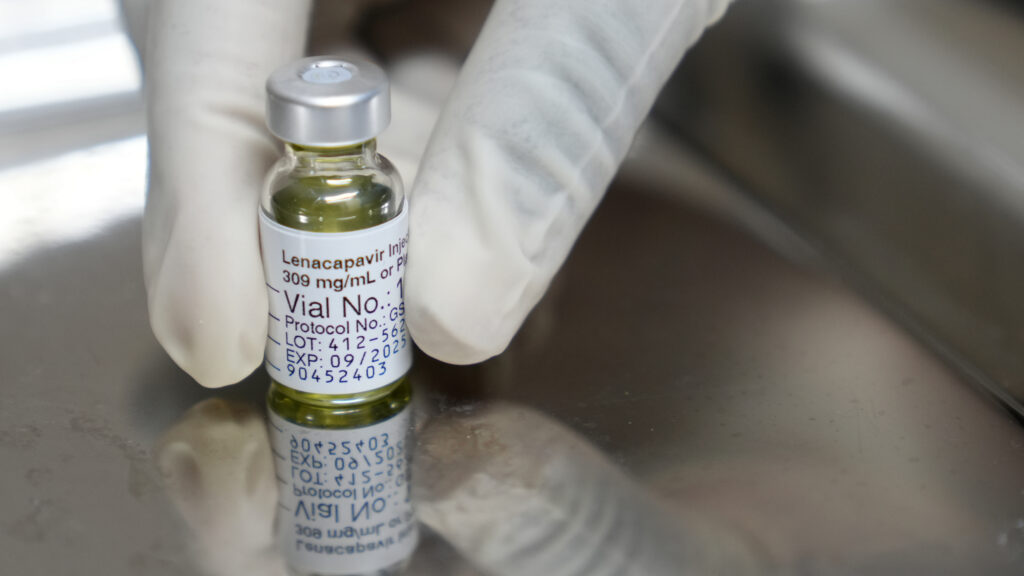
HIV prevention drug distribution begins in Zambia and Eswatini with 1,000 doses, excluding South Africa.
• 1,000 doses of HIV prevention drug delivered to Zambia and Eswatini.
• Distribution excludes South Africa despite high HIV rates.
• Gilead seeks regulatory approval in over a dozen countries.
Strategic Shift
The U.S. Department of State has initiated the distribution of a groundbreaking HIV prevention drug to low-income countries. Approximately 1,000 doses of the Gilead Sciences medication were delivered to Zambia and Eswatini last week. This marks the first delivery under a distribution plan announced late last year. However, the program notably excludes South Africa, despite its high HIV infection rates. The decision has sparked discussions about the strategic priorities of the initiative. Read more on STAT+.
Market Context
Gilead Sciences, the manufacturer of the drug, is providing it at cost to facilitate access in regions heavily impacted by HIV. The company is actively seeking regulatory approval in more than a dozen other sub-Saharan countries. This region remains the epicenter of the AIDS pandemic, with millions affected by the disease. The exclusion of South Africa from the initial distribution has raised questions about the criteria used for selecting recipient countries.
Pipeline Expansion
The drug’s introduction into Zambia and Eswatini is part of a broader effort to expand access to HIV prevention methods in sub-Saharan Africa. Gilead’s ongoing efforts to secure regulatory approvals are crucial for expanding the drug’s availability. The company aims to address the unmet need for effective HIV prevention in regions with high infection rates. Jeremy Lewin, a senior bureau official for foreign assistance, highlighted the initiative as a prime example of leveraging American innovation for global health progress.
Regulatory Pathway
Gilead’s pursuit of regulatory approvals in additional countries is a critical step in broadening the drug’s reach. The company is navigating complex regulatory landscapes to ensure compliance and facilitate access. The success of these efforts will determine the extent to which the drug can impact HIV prevention across sub-Saharan Africa. The initiative underscores the importance of regulatory collaboration in addressing global health challenges.
Patient Impact
The distribution of the HIV prevention drug has significant implications for patients in Zambia and Eswatini. By providing access to effective prevention methods, the initiative aims to reduce new HIV infections and improve public health outcomes. However, the exclusion of South Africa highlights ongoing challenges in ensuring equitable access to healthcare resources. The decision underscores the need for continued advocacy and strategic planning to address disparities in global health initiatives.
For more updates on Market Access, visit our Market Access section.
