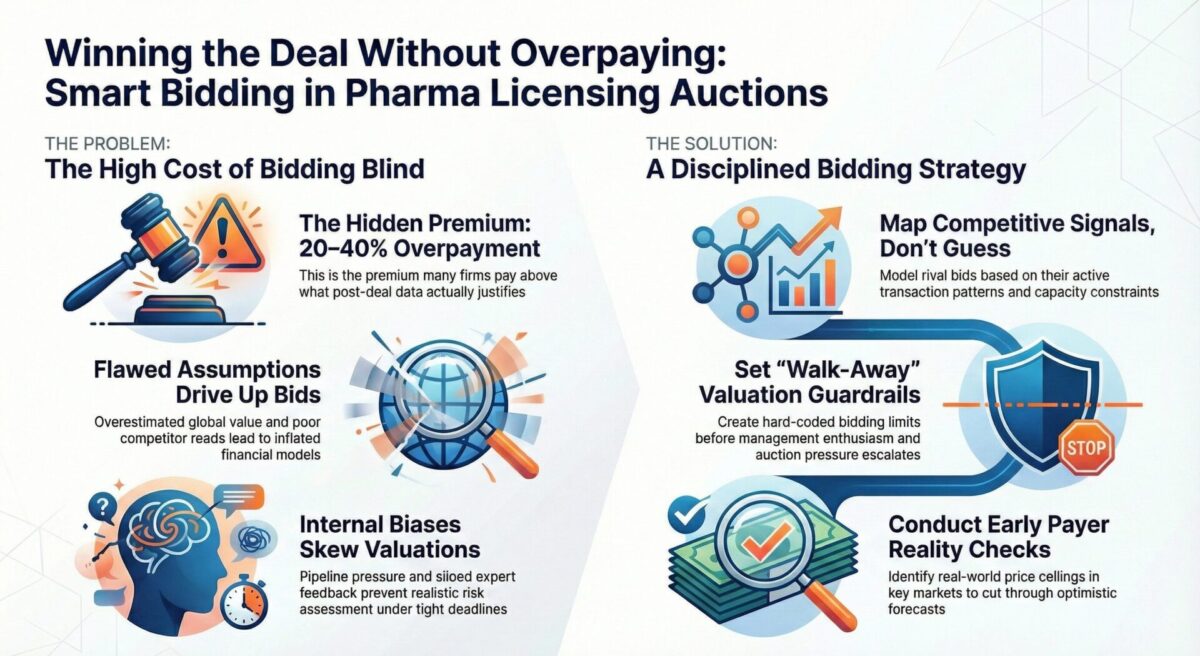
The Hidden Premium Problem
Mid-size pharma companies frequently walk into competitive licensing processes convinced they are bidding rationally, only to discover post-deal that they have paid 20–40% above what later data justified. The challenge is not lack of financial discipline—it is structural blind spots in competitive intelligence, internal incentive systems, and risk translation during accelerated auctions.
Where the Auction Dynamics Break Down
- Shallow competitor reads: BD (Business Development) teams often extrapolate competitor interest from outdated pipeline benchmarks or broad strategic statements rather than active transaction patterns, therapeutic area build-out, or internal capacity constraints of rivals.
- Unvetted assumptions on global expansion value: Mid-size companies frequently overestimate their ability to commercialize outside core regions, inflating projected net present value versus larger bidders who have more realistic country-by-country experience curves.
- Compressed timelines: Seller-driven two-week “best and final” windows eliminate time for cross-functional challenge, leaving financial models untouched by safety, chemistry manufacturing and controls (CMC), or payer reality checks.
The Operational Realities Driving Overpayment
Inside most mid-size organizations, licensing processes accumulate distortions that only surface after final bids are locked:
- BD leadership optimism bias: Pipeline pressure and board expectations subtly shift risk weighting, especially for near-term inflection assets.
- Fragmented input from scientific, regulatory, and market access teams: Early-stage red flags—such as unstable formulation scalability or Health Technology Assessment (HTA) price ceilings—rarely make it into the valuation model with quantified impact.
- Seller-crafted narratives: Auction books emphasize theoretical peak penetration while suppressing real-world payer gatekeeping, forcing bidders to rely on imperfect analogs.
The Commercial Cost of Getting It Wrong
- Deal premium erosion: Within 18–24 months, many mid-size licensees discover that risk-adjusted revenue curves were inflated in high-priority markets. This quickly narrows strategic flexibility for subsequent deals.
- Loss of optionality: Capital tied up in an overpriced asset blocks investment in earlier-platform capabilities or regional expansions.
- Weakened negotiation credibility: Sellers track overpaying patterns and increase reserve prices when mid-size buyers enter future processes.
How Leading BD Teams Prevent Overpayment
High-performing mid-size BD groups apply a triangulation approach that neutralizes auction distortions without slowing execution:
- Competitive signal mapping: Instead of guessing competitor appetite, top performers track in-licensing frequency, modality constraints, recent trial readouts, and shifts in strategic therapeutic area footprints to model realistic rival bids.
- Valuation guardrails: Hard-coded “walk-away bands” are created before management enthusiasm escalates, backed by scenario stress tests from CMC, clinical development, and reimbursement teams.
- Early payer reality checks: Rapid parallel Market Access reads—especially for Germany, France, Japan, and China—cut through optimistic peak share assumptions and identify where HTA downgrades will cap the asset’s real value.
- Auction pace management: Tight internal sprints with pre-defined data challenge templates ensure each new seller update is interrogated, not absorbed uncritically.
The Path Forward
Mid-size pharma can win competitive auctions without overpaying—but only when BD teams replace intuition-driven bidding with structured competitive intelligence discipline, cross-functional scenario friction, and valuation guardrails that cannot be relaxed under deadline pressure. The organizations that systematize these approaches consistently secure assets at rational premiums while maintaining strategic firepower for future deals.
